QUBYX Medical Solutions Inc. announced that LG Electronics selected QUBYX PerfectLum to be bundled with LG medical displays. LG has received FDA 510(K) clearance for the bundle, which will be available immediately.
This combination is the perfect solution for all professionals in the medical field; in particular radiologist, chiropractors, urgent care facilities, hospitals, medical clinics, and all teleradiology providers.
LG Electronics will sell and provide software support for three monitors: The 27-inch 8MP clinical review medical display (27HJ713C-B), the 32-inch 8MP diagnostic display (32HL512D-B), and the 21.3-inch 3MP diagnostic monitor (21HK512D-B)
These displays will now be fully compliant with: Digital Imaging and Communications in Medicine (DICOM) Part 14. LG’s 21HK512D and 32HL512D models have FDA 510(k) Class II approval, and the 27HJ713C model is FDA registered.
Stephen K. Hu, head of medical monitors for LG Business Solutions USA, said:
“As demand for remote healthcare services grows, diagnostic displays will play a crucial role in disciplines such as teleradiology. Together with QUBYX, LG is committed to developing new solutions for healthcare professionals to help them provide the best possible healthcare services.”
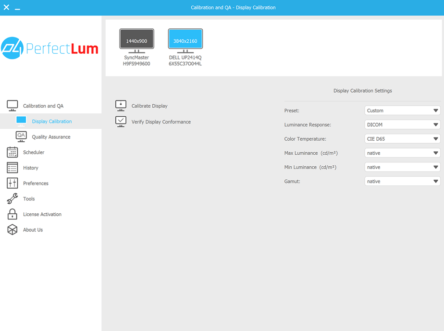
QUBYX’s PerfectLum is a multi-platform, multilingual, full-featured quality assurance tool, with a remote management and cloud solution, that not only calibrates a primary or secondary medical display, but also performs acceptance and constancy tests to verify that displays consistently conform to international regulations including: NEMA DICOM part 14, GSDF, AAPM TG-18, DIN 6868-57, DIN 6868-157, JESRA X-0093, IEC 62563-1 ACR and NYS/NYC Primary Diagnostic Monitor guidelines (NY PDM).